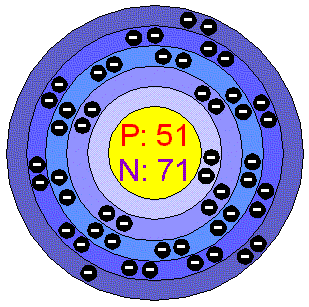
The chemical symbol for antimony is sb. Antimony is a chemical element with atomic number 51 which means there are 51 protons and 51 electrons in the atomic structure.

Atomic number of antimony atomic number of antimony is 51.
Atomic number of antimony. Atomic number of antimony atomic number of antimony is 51. Chemical symbol for antimony is sb. Number of protons in antimony is 51.
Antimony is a chemical element with the symbol sb from latin. Stibium and atomic number 51. A lustrous gray metalloid it is found in nature mainly as the sulfide mineral stibnite sb 2 s 3.
Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics often known by the arabic name kohl. Atomic number of antimony. Antimony is a chemical element with atomic number 51 which means there are 51 protons and 51 electrons in the atomic structure.
The chemical symbol for antimony is sb. Antimony is a chemical element with atomic number 51 which means there are 51 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z.
The total electrical charge of the nucleus is therefore ze where e elementary charge equals to 1 602 x 10 19 coulombs. Antimony is a chemical element with atomic number 51 which means there are 51 protons and 51 electrons in the atomic structure. The chemical symbol for antimony is sb.
Atomic mass of antimony atomic mass of antimony is 121 76 u. 121 76 atomic mass units. 6 684 grams per cubic centimeter.
4 50 per 100 grams. Element antimony sb group 15 atomic number 51 p block mass 121 760. Sources facts uses scarcity sri podcasts alchemical symbols videos and images.
Antimony is a semi metallic chemical element with an atomic number 51 and symbol sb in the periodic table. The latin name of antimony is stibium. It is generally found in two forms namely metallic form and non metallic form.
Antimony compounds are used since ancient times as a component in cosmetics. 121 76 amu melting point. 630 0 c 903 15 k 1166 0 f boiling point.
1750 0 c 2023 15 k 3182 0 f number of protons electrons. 51 number of neutrons. Rhombohedral density 293 k.
6 684 g cm 3 color. Antimony 51 sb occurs in two stable isotopes 121 sb and 123 sb. There are 35 artificial radioactive isotopes the longest lived of which are 125 sb with a half life of 2 75856 years.
124 sb with a half life of 60 2 days. And 126 sb with a half life of 12 35 days. All other isotopes have half lives less than 4 days most less than an hour.