Other forms of the chemical formula for water include d 2 o dho t 2 o and tho. Volume 10 ml 1000 01 l.

Density 1 kg l.
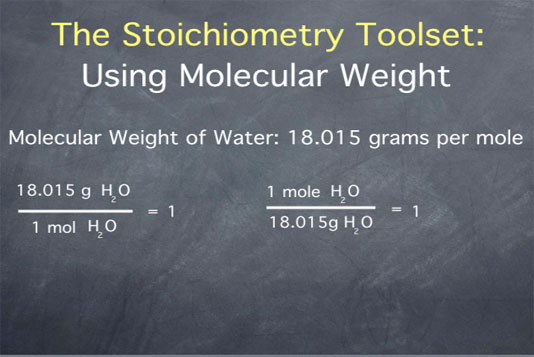
Formula weight of water. Water molecular weight. Molar mass of h2o 18 01528 g mol. Convert grams water to moles or moles water to grams.
1 00794 2 15 9994 percent composition by element. Volume 10 ml 1000 01 l. Density 1 kg l.
Weight 01 kg. Weight 01 kg 1000 10 g. 1 gram is equal to 0 035274 ounces so to get a result in ounces simply multiply the grams by 0 035274.
You can also use our weight conversion calculators to convert from grams and kilograms to pounds and ounces. A cubic foot of water weighs 62 3 pounds at 70 f. The container holds 4 x 4 x 6 96 cubic feet so multiply 96 by 62 3 to get the total weight of the water assuming the container is completely full.
In the si system specific weight of water at 4 c will be. γ 1000 kg m3 9 807 m s2 9807 kg m2 s2 9807 n m3 9 807 kn m3 in the imperial system the mass unit is the slug sl and is derived from the pound force by defining it as the mass that will accelerate at 1 foot per square second when a 1 pound force acts upon it. There are three isotopes of hydrogen.
The usual chemical formula for water assumes the hydrogen atoms consist of the isotope protium one proton no neutrons. Heavy water is also possible in which one or more of the atoms of hydrogen consist of deuterium symbol d or tritium symbol t. Other forms of the chemical formula for water include d 2 o dho t 2 o and tho.